Dass Weihrauchprodukte schon immer und auch heute noch in anderen Kulturen, u.a. Ägypten, zur Behandlung von Husten und Verschleimung angewandt werden, kann man dort auf jedem Kräuterbasar erfahren.
Auch beim Asthma bronchiale handelt es sich um eine Krankheit, bei der Leukotrien eine Rolle spielen. Sie bewirken dort Brochokonstriktion und Bildung von zähem Schleim, was besonders die Ausatmung behindert.
| 1. |
A Supplement with Ribes Nigrum, Boswellia Serrata, Bromelain and Vitamin D to Stop Local Inflammation in Chronic Sinusitis: A Case-Control Study.
De Luca P, D'Ascanio L, Cingolani C, Latini G, Grigaliute E, Di Mauro P, Ralli M, La Mantia I, Di Stadio A
Although chronic sinusitis widely affects the adult population, the treatments currently used did not always satisfactorily solve the symptoms. Traditional therapy with steroids and antibiotics presents risks and benefits and the new drugs, i.e., monoclonal antibody, are valid solutions despite being quite expensive. Natural molecules could be a valid treatment that combines good efficacy and low price. We conducted a case -control study to evaluate the benefit of an oral supplement with Ribes nigrum, Boswellia serrata, Bromelain and Vitamin D on chronic sinusitis symptoms. 60 patients were randomly assigned to one of the three groups: control using nasal steroids only, treatment 1 using nasal steroid and 1 dose of the oral supplement for 30 days and treatment 2 in which patients used nasal steroid and two oral supplement doses daily for 15 days. Conditions of the nasal mucosa and a blood sample (WBC, IgE and CRP) were analyzed at T0, T1 (15 days after treatment) and T2 (30 days after treatment. Patients treated with the supplement improved their nasal findings (hyperemia of mucosa and rhinorrhea) with statistically significant differences from the control. Our preliminary data suggest that the addition of supplement containing Ribes nigrum, Boswellia serrata, Vitamin D and Bromelain to the traditional local therapy (nasal spray with cortisone) can be a supporting therapy to modulate the local inflammation in the nose in patients affected by chronic sinusitis.
J Clin Med. 2023 Apr;12(8):.
PMID: 37109265 [PubMed - as supplied by publisher]
|
| 2. |
Single-Center-Single-Blinded Clinical Trial to Evaluate the Efficacy of a Nutraceutical Containing Boswellia Serrata, Bromelain, Zinc, Magnesium, Honey, Tyndallized Lactobacillus Acidophilus and Casei to Fight Upper Respiratory Tract Infection and Otitis Media.
Della Volpe A, De Luca P, De Lucia A, Martines F, Piroli P, D'Ascanio L, Camaioni A, La Mantia I, Di Stadio A
Some nutraceuticals have been studied as supportive treatment for fighting upper respiratory tract infection and middle ear disease. Our study aims at evaluating the effect of a specific oral supplementation in the treatment of pediatric otits media. The subjects were randomly assigned by the physician (single-blinded study) to one of three groups: Control Group (CG), Treatment Group 1 (TG1), or Treatment Group 2 (TG2). Both TG were treated with Flogostop Duo (for 20 days-TG1 or 30 days-TG2) in combination with the standard treatment, while CG underwent standard treatment only. The standard treatment was nasal aerosol with Fluticasone and Mucolytic, and nasal washing with hypertonic solution. All patients were analyzed by otoscopy, impedance, fibroscopy, and pure auditory test at the baseline (T0), after 20 days (T1) and 35 days (T2). 120 children were included in the study, 40 in the CG, 40 in the TG1, and 40 in the TG2. Both TG1 and TG2 presented statistically significant differences with respect to controls in otoscopy, impedance, fibroscopy, and PTA at T2. The otoscopy improved at T2 with statistically significant value only in TG2. The impedance and fibroscopy improved at T1 both in TG1 and TG2 compared to CG. A statistically significant improvement was observed in TG2 at T2 in comparison to both CG and TG1. Statistically significant differences were observed in PTA at T2 only compared with controls. This study confirmed the efficacy of nutraceutical as supporting therapy in the upper respiratory tract infection in children. In particular, the supplement containing Boswellia serrata and Bromelain, which are molecules with strong anti-inflammatory and pain-control capacities, could add the benefit without the adverse effects which are related to NSAID use.
Healthcare (Basel). 2022 Aug;10(8):.
PMID: 36011184 [PubMed - as supplied by publisher]
|
| 3. |
Aromatherapy blend of thyme, orange, clove bud, and frankincense boosts energy levels in post-COVID-19 female patients: A randomized, double-blinded, placebo controlled clinical trial.
Hawkins J, Hires C, Keenan L, Dunne E
BACKGROUND: A large proportion of individuals who have recovered from an acute COVID-19 infection continue to experience symptoms months later. Post-acute COVID-19 (long-haul COVID-19) can range from serious complications to quality of life symptoms such as fatigue or insomnia. The purpose of this study was to evaluate the potential for inhalation of essential oils to improve energy levels among otherwise healthy female survivors of acute COVID-19 who experience a lack of energy more than five months after recovery. This study was conducted in the United States in late 2021.
METHOD: This was a randomized double blind, placebo controlled trial to evaluate the potential for inhalation of Longevity™, a proprietary essential oil blend manufactured by Young Living Essential Oils (Lehi, Utah, USA), on energy levels among female survivors of COVID-19 who continue to experience fatigue more than 5 months recovery from the acute infection. Forty women were randomized to two groups: intervention and placebo. Both groups inhaled the assigned product twice daily for fourteen consecutive days. Fatigue scores were measured using the Multidimensional Fatigue Symptom Inventory (MFSI). Secondary outcomes included scores on each of the MFSI's ten subscales.
RESULTS: Individuals who inhaled the essential oil blend for 2 weeks had significantly lower fatigue scores after controlling for baseline scores, employment status, BMI, olfactory function, and time since diagnosis, with a large effect size (F (1,39) = 6.15, p = .020, partial eta squared = 0.198). Subscale analysis identified subscales of vigor, as well as global, behavioral, general, and mental fatigue as benefiting from the intervention. This study provides evidence that a proprietary aromatherapy blend can significantly improve energy levels among women who are experiencing fatigue after recovering from COVID-19.
Complement Ther Med. 2022 Aug;67():102823.
PMID: 35341944 [PubMed - indexed for MEDLINE]
|
| 4. |
Inflawell improves neutrophil-to-lymphocyte ratio and shortens hospitalization in patients with moderate COVID-19, in a randomized double-blind placebo-controlled clinical trial.
Barzin Tond S, Balenci L, Khajavirad N, Salehi M, Tafakhori A, Shahmohammadi MR, Ghiasvand F, Jafari S, Abolghasemi S, Mokhtari F, Mahmoodi Baram S, Zarei T, Kazemi D, Mohammadnejad E, Shah-Hosseini A, Haghbin Toutounchi A, Fallah S, Riazi A, Karima S
AIMS: COVID-19 is a significant global threat to public health. Despite the availability of vaccines and anti-viral drugs, there is an urgent need for alternative treatments to help prevent and/or manage COVID-19 symptoms and the underlying dysregulated immune response. We hypothesized that administration of Inflawell syrup, a Boswellia extract formulation enriched for boswellic acids (BAs), can reduce the excessive or persistent inflammation and thereby prevent disease progression. BAs are medicinally activated triterpenoids found in the resins of Boswellia spp., and possess an immense therapeutic potential due to their anti-inflammatory and immunoregulatory activities. We investigated the effect of Inflawell syrup, on moderate COVID-19 patients along with the current standard of care treatment.
METHODS: A randomized placebo-controlled double-blind clinical trial was conducted, following definitive confirmation of COVID-19. Forty-seven hospitalized patients with moderate COVID-19 were enrolled and received either the Inflawell syrup or placebo. Clinical symptoms and markers of inflammation were evaluated at baseline and completion of the trial.
RESULTS: Our clinical trial revealed an increase in the percentage of oxygen saturation level in patients that received the BAs compared to placebo (P < 0.0001). In addition, the average duration of hospitalization was significantly shorter in the BAs group compared with the placebo group (P < 0.04). Concomitantly, some improvement in the clinical symptoms including cough, dyspnea, myalgia, headache, and olfactory and gustatory dysfunction were detected in the BAs group. Hematologic findings showed a significant decrease in the percentage of neutrophils (P < 0.006) and neutrophil-to-lymphocyte ratio (NLR) levels (P < 0.003), associated with a significant increase in the percentage of lymphocytes in the BAs group compared with the placebo (P < 0.002). Additionally, a significant decrease in CRP, LDH, IL - 6 and TNF - α levels was detected in the BAs group. Following the intervention, fewer patients in the BAs group were PCR-positive for COVID-19 compared to placebo, though not statistically significant.
CONCLUSION: Overall, the treatment with Inflawell resulted in shorter hospital stay, alleviation of COVID-19 clinical symptoms and decline in the level of pro-inflammatory cytokines.
TRIAL REGISTRATION: The trial has been registered in https://www.irct.ir with unique identifier: IRCT20170315033086N10 ( https://en.irct.ir/trial/51631 ). IRCT is a primary registry in the WHO registry network ( https://www.who.int/clinical-trials-registry-platform/network/primary-registries ).
Inflammopharmacology. 2022 Apr;30(2):465-475.
PMID: 35201518 [PubMed - indexed for MEDLINE]
|
| 5. |
Effectiveness of complementary therapies for the management of symptom clusters in palliative care in pediatric oncology: a systematic review.
Lopes-Júnior LC, Urbano IR, Schuab SIPC, Pessanha RM, Rosa GS, Lima RAG
OBJECTIVE: To evaluate the effectiveness of complementary therapies in the management of symptom clusters in children and adolescents with cancer undergoing palliative care.
METHOD: Systematic review guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, resorting to the databases MEDLINE, Web of Science, Central Cochrane, and PsycINFO. The identification, selection, inclusion, extraction, and methodological assessment were conducted by two independent reviewers.
RESULTS: Five quasi-experiments met the eligibility criteria. The heterogeneous characteristics of the studies made meta-analysis impossible. Two studies used therapeutic massage, one used Reiki, one used boswellic acid, and one used Cannabis sativa; three of them (therapeutic massage and Reiki) presented statistically significant results for the management of the cluster pain-anxiety-worry-dyspnea. Most studies presented a moderate risk of bias as per ROBINS-I tool.
CONCLUSION: Therapeutic massage and Reiki may be effective for the symptom clusters management, especially the pain-anxiety-worry-dyspnea cluster in children and adolescents undergoing palliative care.
Rev Esc Enferm USP. 2021;55():03709.
PMID: 34037196 [PubMed - indexed for MEDLINE]
|
| 6. |
Multi-center, randomized, double-blind, placebo-controlled, exploratory study to evaluate the efficacy and safety of HAD-B1 for dose-finding in EGFR positive and locally advanced or metastatic NSCLC subjects who need Afatinib therapy: Study protocol clinical trial (SPIRIT Compliant).
Park SJ, Kang HJ, Jun HJ, Shin SH, Yoo HS
BACKGROUND: In recent studies, afatinib, a second-generation inhibitor, showed superior outcomes, when compared to the first-generation of EGFR-tyrosine kinase inhibitors (TKIs), such as erlotinib and gefitinib, in patients with advanced non-small cell lung cancer (NSCLC) harboring mutations of epidermal growth factor receptor (EGFR). Patients who receive TKIs with a significant initial efficacy, inevitably experience an acquired resistance (AR) within 9 to 13 months. Traditional Korean medicine may have synergistic effects when combined with chemotherapy or radiotherapy. The purpose of this trial is to assess whether afatinib plus HAD-B1 improves disease control rates (DCRs) compared with afatinib alone and to evaluate the efficacy and safety of HAD-B1 for finding the proper dose.
METHODS: This is a randomized, double-blind, placebo-controlled, multi-center, therapeutic, exploratory clinical trial. This trial is designed to determine whether HAD-B1 combined with afatinib results in better DCRs with less toxicity than afatinib alone. A total of 66 NSCLC patients with EGFR mutations will be randomly assigned to treatment group 1 (afatinib 40 mg/day plus HAD-B1 972 mg), treatment group 2 (afatinib 40 mg/day plus HAD-B1 1944 mg) and a control group (afatinib 40 mg/day). Afatinib combined with HAD-B1 or with a placebo will be administered to the participants for 12 weeks. The primary endpoint is a comparison of the DCRs among groups. Secondary endpoints are comparisons of the complete response (CR) and the partial response (PR) to the treatment, the stability of the disease (SD), progression free survival (PFS), time to progression (TTP), and tumor marker (CEA, NSE) and WBC differential count (LMR, NLR) and natural killer cell activity and quality of life (QOL) among groups.
DISCUSSION: The results from this clinical trial will provide evidence of efficacy and safety of HAD-B1 in EGFR positive and locally advanced or metastatic NSCLC patients who need afatinib therapy.
Medicine (Baltimore). 2020 Jan;99(4):e18735.
PMID: 31977865 [PubMed - indexed for MEDLINE]
|
| 7. |
A novel herbal composition containing extracts of Boswellia serrata gum resin and Aegle marmelos fruit alleviates symptoms of asthma in a placebo controlled double-blind clinical study.
Yugandhar P, Rao KM, Sengupta K
LI13109F is a novel herbal composition containing the extracts of Boswellia serrata gum resin and Aegle marmelos fruit. This composition dampens leukotriene dependent inflammatory reactions via inhibiting 5-lipoxygenase pathway. In a Sephadex LH-20 induced airway inflammation model of Sprague Dawley rats, LI13109F significantly reduced infiltrated granulocyte population in the bronco-alveolar lavage fluid and normalized Th1/Th2 cytokine balance. Further, a 56-day placebo-controlled and randomized double blind study (Clinical Trial Registration No. CTRI/2016/10/007393) on subjects with mild to moderate asthma has evaluated the clinical efficacy of LI13109F. The study subjects received either 200 mg/day of LI13109F (n = 18) or a similar dosage of placebo (n = 18). At the end of the trial period, LI13109F conferred significant improvements in the clinical parameters; the emotional function (p = .0305) and asthma symptoms scores (p = .0002) were improved even at 14 days, compared with the placebo. Further, 56 days supplementation of LI13109F resulted in significant increase in serum IFN-γ (p = .0014) and reduction in IL-4 (p = .0497), compared with placebo. LI13109F supplementation did not yield any serious adverse events or any abnormal observations in routine laboratory examinations during the study. Together, these observations suggest that LI13109F (AlvioLife®) is tolerable and an effective intervention for management of mild to moderate asthma such as airway inflammation.
Phytother Res. 2018 Jan;32(1):140-150.
PMID: 29210124 [PubMed - indexed for MEDLINE]
|
| 8. |
Medicinal Plants for the Treatment of Asthma: A Traditional Persian Medicine Perspective.
Javadi B, Sahebkar A, Emami SA
OBJECTIVE: To search major Traditional Persian Medicine (TPM) textbooks for medicinal plants used to treat asthma. The conformity of the TPM findings on the anti-asthmatic efficacy of plants with the findings of pharmacological studies was also explored.
METHODS: Major TPM textbooks were hand searched to find medicinal plants used for the treatment of asthma. Scientific names of TPM-suggested plants were determined using botanical databases and were used for a multidatabase electronic search in PubMed, Scopus, ScienceDirect and Google Scholar databases. Then, the antiasthmatic effectiveness of TPM-recommended plants was verified in view of the findings from modern pharmacological investigations.
RESULTS: According to the main TPM texts, Adianthum capillus-veneris, Boswellia oleogumresin, Crocus sativus, Glycyrrhiza glabra, Hyssopus officinalis and Ruta graveolens were the most efficacious medicinal plants for the treatment of asthma. This finding was confirmed by pharmacological studies which showed counterbalancing effects of the above-mentioned plants on inflammation, oxidative stress, allergic response, tracheal smooth muscle cell constriction and airway remodeling.
CONCLUSION: The strong ethnobotanical background of plants used in TPM could be a valuable tool to find new anti-asthmatic medications. In this review, TPM-suggested anti-asthmatic plants were found to possess several mechanisms relevant to the treatment of respiratory diseases according to the information retrieved from modern pharmacological studies. This high degree of conformity suggested further proof-of-concept trials to ascertain the role of these plants in the routine management of asthmatic patients.
Curr Pharm Des. 2017;23(11):1623-1632.
PMID: 27774904 [PubMed - indexed for MEDLINE]
|
| 9. |
Functional study on Boswellia phytosome as complementary intervention in asthmatic patients.
Ferrara T, De Vincentiis G, Di Pierro F
OBJECTIVE: The combination of inhaled corticosteroids (ICS) and long-acting beta-agonists (LABAs) is recommended for the treatment of patients with mild-to-severe persistent asthma. However, given the lack of definite and safe therapies, complementary or alternative medicines are frequently used by asthmatic patients in combination with standard treatments.
PATIENTS AND METHODS: A group of asthmatic subjects have been enrolled in this multicenter study; after having verified the compliance to their current medical therapy (ICS + LABAs), the subjects have been randomized to receive Casperome® 500 mg/day or no additional treatment for a period of 4 weeks. They were also asked to keep track of the number of inhalations required per day and any adverse events through a daily form.
RESULTS: A total of 32 subjects were enrolled in the study. Subjects receiving Casperome® 500 mg/day in addition to the standard ICS + LABAs treatment showed a decrease in the number of inhalations needed compared to patients who did not receive Casperome® therapy. The treatment was well tolerated and only mild-moderate adverse events were registered.
CONCLUSIONS: The use of Casperome® 500 mg/day is beneficial for asthmatic patients as it helps reduce the need for inhalation therapy with ICS + LABA.
Eur Rev Med Pharmacol Sci. 2015 Oct;19(19):3757-62.
PMID: 26502867 [PubMed - indexed for MEDLINE]
|
| 10. |
Holy Saturday asthma.
O'Connor TM, Cusack R, Landers S, Bredin CP
A 61-year-old man complained of cough and dyspnoea after exposure to colophony-containing solder fumes at work. A histamine challenge test confirmed airway hyper-responsiveness, and colophony-challenge demonstrated a 16.7% drop in peak expiratory flow rate (PEFR), supporting a diagnosis of colophony-induced occupational asthma. At review, the patient presented with cough, dyspnoea and wheeze that occurred acutely when exposed to the fumes from burning incense during Easter Saturday services, necessitating his departure from the church. Inhalation challenge tests using two blends of incense used at his church (Greek and Vatican) led to identical symptoms and a significant reduction in forced expiratory volume in 1 s 15 min after exposure and PEFRs up to 48 h after exposure, indicating an early and late phase asthmatic reaction. This is the first report of coexistent colophony and incense-induced asthma. The similarities in chemical structures between abietic acid in colophony and boswellic acid in incense suggest a common mechanism.
BMJ Case Rep. 2014 Mar;2014():.
PMID: 24626388 [PubMed - indexed for MEDLINE]
|
| 11. |
Natural anti-inflammatory products and leukotriene inhibitors as complementary therapy for bronchial asthma.
Houssen ME, Ragab A, Mesbah A, El-Samanoudy AZ, Othman G, Moustafa AF, Badria FA
OBJECTIVE: To assess the efficacy of a combination of Boswellia serrata, licorice root (Glycyrrhiza glabra) and Tumeric root (Curcuma longa) as natural leukotriene inhibitor, antiinflammatory and antioxidant products respectively in controlling bronchial asthma.
SUBJECTS AND METHODS: The study comprised 63 patients with bronchial asthma that are further subdivided into two groups .Group 1 receiving oral capsule (combined herb) in a soft-gelatin capsule 3 times daily for 4weeks and group 2 receiving placebo. Plasma leukotriene C(4) (LTC(4))(,) nitric oxide (NO) and malondialdehyde (MDA) levels were measured and pulmonary function was also assessed in all patients enrolled in the study.
RESULTS: There was a statistically significant decrease in the plasma levels of LTC(4), (MDA), and NO in target therapy group when compared with placebo group.
CONCLUSION: The used extract contained Boswellia serrata, Curcuma longa and Glycyrrhiza has a pronounced effect in the management of bronchial asthma.
Clin Biochem. 2010 Jul;43(10-11):887-90.
PMID: 20430018 [PubMed - indexed for MEDLINE]
|
| 12. |
Respiratory and allergic diseases: from upper respiratory tract infections to asthma.
Jaber R
Patients with asthma and allergic rhinitis may benefit from hydration and a diet low in sodium, omega-6 fatty acids, and transfatty acids, but high in omega-3 fatty acids (i.e., fish, almonds, walnuts, pumpkin, and flax seeds), onions, and fruits and vegetables (at least five servings a day). Physicians may need to be more cautious when prescribing antibiotics to children in their first year of life when they are born to families with a history of atopy. More research is needed to establish whether supplementation with probiotics (lactobacillus and bifidobacterium) during the first year of life or after antibiotic use decreases the risk of developing asthma and allergic rhinitis. Despite a theoretic basis for the use of vitamin C supplements in asthmatic patients, the evidence is still equivocal, and long-term studies are needed. The evidence is stronger for exercise-induced asthma, in which the use of vitamin C supplementation at a dosage of 1 to 2 g per day may be helpful. It is also possible that fish oil supplements, administered in a dosage of 1 to 1.2 g of EPA and DHA per day, also may be helpful to some patients with asthma. Long-term studies of fish oil and vitamin C are needed for more definite answers. For the patient interested in incorporating nutritional approaches, vitamin C and fish oils have a safe profile. However, aspirin-sensitive individuals should avoid fish oils, and red blood cell magnesium levels may help in making the decision whether to use additional magnesium supplements. Combination herbal formulas should be used in the treatment of asthma with medical supervision and in collaboration with an experienced herbalist or practitioner of TCM. Safe herbs, such as Boswellia and gingko, may be used singly as adjuncts to a comprehensive plan of care if the patient and practitioner have an interest in trying them while staying alert for drug-herb interactions. No data on the long-term use of these single herbs in asthma exist. For the motivated patient, mind-body interventions such as yoga, hypnosis, and biofeedback-assisted relaxation and breathing exercises are beneficial for stress reduction in general and may be helpful in further controlling asthma. Encouraging parents to learn how to massage their asthmatic children may appeal to some parents and provide benefits for parents and children alike. Acupuncture and chiropractic treatment cannot be recommended at this time, although some patients may derive benefit because of the placebo effect. For patients with allergic rhinitis, there are no good clinical research data on the use of quercetin and vitamin C. Similarly, freeze-dried stinging nettle leaves may be tried, but the applicable research evidence also is poor. Further studies are needed to assess the efficacy of these supplements and herbs. Homeopathic remedies based on extreme dilutions of the allergen may be beneficial in allergic rhinitis but require collaboration with an experienced homeopath. There are no research data on constitutional homeopathic approaches to asthma and allergic rhinitis. Patients with COPD are helped by exercise, pulmonary rehabilitation, and increased caloric protein and fat intake. Vitamin C and n-3 supplements are safe and reasonable; however, studies are needed to establish their efficacy in COPD. On the other hand, there are convincing data in favor of N-acetyl-cysteine supplementation for the patient with COPD at doses ranging between 400 and 1200 mg daily. Red blood cell magnesium levels may guide the use of magnesium replacement. The use of L-carnitine and coenzyme Q10 in patients with COPD needs further study. The addition of essential oils to the dietary regimen of patients with chronic bronchitis is worth exploring. Patients with upper respiratory tract infections can expect a shorter duration of symptoms by taking high doses of vitamin C (2 g) with zinc supplements, preferably the nasal zinc gel, at the onset of their symptoms. Adding an herb such as echinacea or Andrographis shortens the duration of the common cold. The one study on Elderberry's use for the flu was encouraging, and the data on the homeopathic remedy Oscillococcinum interesting, but more studies should be performed. Saline washes may be helpful to patients with allergic rhinitis and chronic sinusitis. Patients also may try the German combination (available in the United States) of elderberry, vervain, gentian, primrose, and sorrel that has been tested in randomized clinical trials. Bromelain is safe to try; the trials of bromelain supplementation were promising but were never repeated. The preceding suggestions need to be grounded in a program based on optimal medical management. Patients need to be well educated in the proper medical management of their disease and skilled at monitoring disease stability and progress. Asthmatic patients need to monitor their bronchodilator usage and peak flow meter measurements to step up their medical treatment in a timely manner, if needed. Patients welcome physician guidance when exploring the breadth of treatments available today. A true patient-physician partnership is always empowering to patients who are serious about regaining their function and health.
Prim Care. 2002 Jun;29(2):231-61.
PMID: 12391710 [PubMed - indexed for MEDLINE]
|
| 13. |
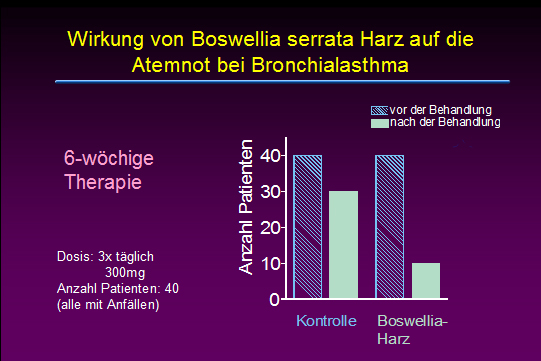
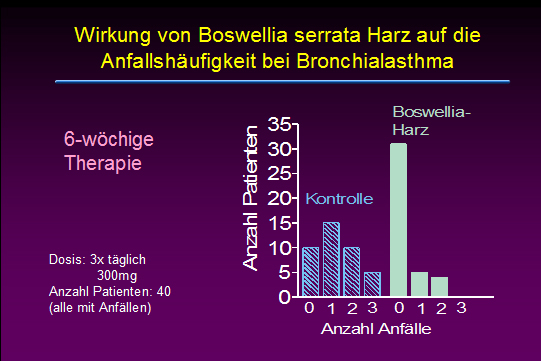
Effects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study.
Gupta I, Gupta V, Parihar A, Gupta S, Lüdtke R, Safayhi H, Ammon HP
The gum resin of Boswellia serrata, known in Indian Ayurvedic system of medicine as Salai guggal, contains boswellic acids, which have been shown to inhibit leukotriene biosynthesis. In a double-blind, placebo-controlled study forty patients, 23 males and 17 females in the age range of 18 - 75 years having mean duration of illness, bronchial asthma, of 9.58 +/- 6.07 years were treated with a preparation of gum resin of 300 mg thrice daily for a period of 6 weeks. 70% of patients showed improvement of disease as evident by disappearance of physical symptoms and signs such as dyspnoea, rhonchi, number of attacks, increase in FEV subset1, FVC and PEFR as well as decrease in eosinophilic count and ESR. In the control group of 40 patients 16 males and 24 females in the age range of 14-58 years with mean of 32.95 +/- 12.68 were treated with lactose 300 mg thrice daily for 6 weeks. Only 27% of patients in the control group showed improvement. The data show a definite role of gum resin of Boswellia serrata in the treatment of bronchial asthma.
Eur J Med Res. 1998 Nov;3(11):511-4.
PMID: 9810030 [PubMed - indexed for MEDLINE]
|