Bei chronisch entzündlichen Darmerkrankungen findet man in der Lavage des Darmes erhebliche Mengen an Leukotrien. Mehrere Studien und Hunderte von Einzelbeobachtungen konnten inzwischen eine Verbesserung (keine Heilung) der Situation bei einschlägigen Patienten erzielen.
| 1. |
The Role of Plant-Derived Natural Products in the Management of Inflammatory Bowel Disease-What Is the Clinical Evidence So Far?
Davila MM, Papada E
Inflammatory bowel diseases (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), are a major healthcare challenge worldwide. Disturbances in the immune system and gut microbiota followed by environmental triggers are thought to be part of the aetiological factors. Current treatment for IBD includes corticosteroids, immunosuppressants, and other biologic agents; however, some patients are still unresponsive, and these are also linked to high financial load and severe side effects. Plant-derived natural products are rich in phytochemicals and have been used as healing agents in several diseases since antiquity due to their antioxidant, anti-inflammatory, and immunomodulatory properties, as well as gut microbiota modulation. Numerous in vitro and in vivo studies have shown that phytochemicals act in key pathways that are associated with the pathogenesis of IBD. It is also reported that the use of plant-derived natural products as complementary treatments is increasing amongst patients with IBD to avoid the side effects accompanying standard medical treatment. This review summarises the relevant evidence around the use of plant-derived natural products in the management of IBD, with specific focus on the clinical evidence so far for Curcumin, Mastiha, and .
Life (Basel). 2023 Aug;13(8):.
PMID: 37629560 [PubMed - as supplied by publisher]
|
| 2. |
Effect of an herbal mouthwash on periodontal indices in patients with plaque-induced gingivitis: A cross-over clinical trial.
Talebi Ardakani M, Farahi A, Mojab F, Moscowchi A, Gharazi Z
Recent advances in alternative medicine have led to the introduction of various new herbal products for treating gingivitis as the most prevalent gingival disease. The present study clinically evaluated the effect of a herbal mouthwash consisting of 5 herbal extracts (Myrtus communis, Quercus brantii, Punica granatum, Portulaca olerace, and Boswellia serrata) on periodontal indices. Fifty patients with plaque-induced gingivitis were included in this randomized, dou-ble-blinded clinical trial and divided into two groups. Following scaling and root planing (SRP), they were prescribed 0.2% chlorhexidine (CHX) (group 1) and herbal mouthwash (group 2) twice a day for 14 days. Both groups received saline mouthwash for the subsequent 14 days (wash-out time). Then, they used the mouthwashes in a cross-over manner for an additional two weeks. Probing pocket depth (PPD), gingival index (GI), bleeding on probing (BOP), and plaque index (PI) were recorded at baseline and after each period of mouthwash use. The data were analyzed by SPSS software, using generalized estimating equation (GEE) linear regression and paired t-test. P<0.05 was considered sta-tistically significant. Both groups exhibited statistically significant improvements in the periodontal indices compared to the baseline (P<0.05); however, there were no significant differences between the two study groups in this regard. The experimental herbal mouthwash improved the periodontal condition in plaque-induced gingivitis after two weeks, comparable to the effect of %0.2 CHX mouthwash in terms of PPD, BOP, PI, and GI.
J Adv Periodontol Implant Dent. 2022;14(2):109-113.
PMID: 36714089 [PubMed - as supplied by publisher]
|
| 3. |
Complementary medicines used in ulcerative colitis and unintended interactions with cytochrome P450-dependent drug-metabolizing enzymes.
Şen A
Ulcerative colitis (UC) is an idiopathic, chronic inflammatory disease with multiple genetic and a variety of environmental risk factors. Although current drugs significantly aid in controlling the disease, many people have led to the application of complementary therapies due to the common belief that they are natural and safe, as well as due to the consideration of the side effect of current drugs. Curcumin, cannabinoids, wheatgrass, Boswellia, wormwood and Aloe vera are among the most commonly used complementary medicines in UC. However, these treatments may have adverse and toxic effects due to unintended interactions with drugs or drug-metabolizing enzymes such as cytochrome P450s; thus, being ignorant of these interactions might cause deleterious effects with severe consequences. In addition, the lack of complete and controlled long-term studies with the use of these complementary medicines regarding drug metabolism pose additional risk and unsafety. Thus, this review aims to give an overview of the potential interactions of drug-metabolizing enzymes with the complementary botanical medicines used in UC, drawing attention to possible adverse effects.
Turk J Med Sci. 2022 Oct;52(5):1425-1447.
PMID: 36422483 [PubMed - indexed for MEDLINE]
|
| 4. |
Positive Effects of a Lecithin-Based Delivery Form of Extract in Acute Diarrhea of Adult Subjects.
Giacosa A, Riva A, Petrangolini G, Allegrini P, Fazia T, Bernardinelli L, Peroni G, Rondanelli M
Acute diarrhea is a frequent problem worldwide, mostly due to gastrointestinal infections or food poisoning. Boswellia serrata could be active in the treatment of acute diarrhea due to its anti-inflammatory, antispasmodic, and antimicrobial activity. In this randomized, double-blind, placebo-controlled clinical study, 49 adults with acute diarrhea were randomly allocated to receive 250 mg of a lecithin-based delivery form of Boswellia serrata (CASP) or placebo for 5 days. The time it took to become healthy with stoppage of diarrhea (primary end point) was significantly shorter in the intervention group (3.08 vs. 4.44 days: p-value < 0.0001). The probability of subjects treated with CASP to recover sooner was equal to 80.2%. A significantly lower number of stools was observed in the CASP group over time (β = −0.17, p-value < 0.0001). A significant difference was observed between the two groups for abdominal pain, nausea, and GAE (global assessment of efficacy). In conclusion, the lecithin-based delivery form of Boswellia serrata extract could be a useful addition to the treatment of acute diarrhea in adults. CASP is safe and reduces the time it takes to become healthy, the frequency of stools, the abdominal pain and nausea of subjects with acute diarrhea. Further studies are needed to confirm these promising results.
Nutrients. 2022 Apr;14(9):.
PMID: 35565826 [PubMed - indexed for MEDLINE]
|
| 5. |
The effectiveness of olibanum orally disintegrating tablet in the treatment of oral aphthous ulcers: A randomized, double-blind, placebo-controlled clinical trial.
Soltani R, Saberi Z, Ghanadian SM, Taheri A, Entezarhojjat A
BACKGROUND: oleo-gum-resin (frankincense; olibanum) has anti-inflammatory, analgesic, and antimicrobial effects. This study aimed to evaluate the clinical effectiveness of frankincense extract in the treatment of oral aphthous ulcers.
MATERIALS AND METHODS: In a randomized, double-blind, placebo-controlled clinical trial, patients with aphthous ulcers were randomly assigned to either experimental (Frankincense extract) or placebo groups to use orally disintegrating tablets (ODT) of frankincense and placebo, respectively, four times a day for 3 days. The size of aphthous ulcers and the pain severity by visual analogue scale were recorded at days 0, 2, and 4 and compared between the groups.
RESULTS: Twenty-five patients in each group completed the study. Olibanum extract ODT significantly reduced the ulcer size on the second ( < 0.001) and fourth ( < 0.001) days as well as the pain score on the second ( = 0.002) and fourth ( < 0.001) days of the intervention compared to placebo. Furthermore, at the end of the intervention, the number of patients with complete ulcer healing and pain relief in the experimental group was significantly more than the placebo group (5 vs. 0, = 0.02; and 11 vs. 0, < 0.001, respectively).
CONCLUSION: Taking olibanum extract ODTs reduces the ulcer size and pain severity and accelerates the healing process in the oral aphthous lesions.
J Res Med Sci. 2022;27():8.
PMID: 35342437 [PubMed - as supplied by publisher]
|
| 6. |
Beneficial Effects on Abdominal Bloating with an Innovative Food-Grade Formulation of and Extracts in Subjects with Irritable Bowel Syndrome and Small Bowel Dysbiosis.
Giacosa A, Riva A, Petrangolini G, Allegrini P, Fazia T, Bernardinelli L, Peroni G, Rondanelli M
Bloating is a symptom frequently reported by subjects with irritable bowel syndrome (IBS) and small bowel dysbiosis, and Low FODMAP’s diet (LFD) has been used to treat them. Extracts of Curcumalonga and Boswelliaserrata share anti-inflammatory and antimicrobial effects that could be useful in the management of these clinical conditions. The aim of this study was to evaluate the efficacy of curcumin and boswellia extracts (as Curcumin Boswellia Phytosome, CBP) and LFD on the relief of abdominal bloating in IBS subjects with small bowel dysbiosis, in comparison to LFD alone, in a 30-day supplementation, randomized trial. IBS participants were randomized to either the intervention (500 mg bid of CBP and LFD) or control arm (LFD). Small bowel dysbiosis has been defined by an increase of urinary indican with normal urinary skatole. A total of 67 subjects were recruited. The intervention group (33 subjects) showed a significant decrease (p < 0.0001) of bloating, abdominal pain, and indican values at the end of the study, when compared to the control group (34 subjects). Moreover, the subjects of the intervention group showed a significantly better (p < 0.0001) global assessment of efficacy (GAE) as compared to controls. In conclusion, in subjects with IBS and small bowel dysbiosis, abdominal bloating can be successfully reduced with a supplementation with CBP and LFD.
Nutrients. 2022 Jan;14(3):.
PMID: 35276778 [PubMed - indexed for MEDLINE]
|
| 7. |
Natural compounds as safe therapeutic options for ulcerative colitis.
Gupta M, Mishra V, Gulati M, Kapoor B, Kaur A, Gupta R, Tambuwala MM
Ulcerative colitis (UC) is a chronic inflammatory bowel disease of unknown etiology. Several conventional treatments for UC such as corticosteroids, immunosuppressive agents, tumor necrosis factor antagonist, integrin blockers, and interleukin antagonist, and salicylates are available but are associated with the various limitations and side-effects. None of the above treatments helps to achieve the ultimate goal of the therapy, i.e., maintenance of remission in the long-term. Natural remedies for the treatment of UC show comparatively less side effects as compared to conventional approaches, and affordable. The current review presents details on the role of herbal drugs in the treatment and cure of UC. Google, PubMed, Web of Science, and Scopus portals have been searched for potentially relevant literature to get the latest developments and updated information related to use of natural drugs in the treatment of UC. Natural products have been used over centuries to treat UC. Some of the essential herbal constituents exhibiting antiulcerogenic activity include gymnemic acid (Gymnema sylvestre), shagoal (Zingiber officinale), catechin (Camellia sinensis), curcumin (Curcuma longa), arctigenin (Arctium lappa), and boswellic acid (Boswellia serrata). Although many plant-derived products have been recommended for UC, further research to understand the exact molecular mechanism is still warranted to establish their usefulness clinically.
Inflammopharmacology. 2022 Apr;30(2):397-434.
PMID: 35212849 [PubMed - indexed for MEDLINE]
|
| 8. |
Efficacy of and based with orally in the management of w.s.r. to fistula-in-ano: A open labelled randomized comparative clinical study.
Nema A, Gupta SK, Dudhamal T, Mahanta V
BACKGROUND: is a disease of ano-rectal region and can be correlated with fistula-in-ano. (application of medicated thread) is being practiced for ano-rectal disorders, particularly in . -based has shown good results in previous studies. Literatures and experiments of showed anti-inflammatory, antifungal, analgesic, wound healing properties and (resin of Roxb.) is also having binding effect. Here, -based is used in comparison of -based with orally for better outcome in the management of .
AIM: The aim of this study was to evaluate and compare the efficacy of and based with orally in the management of .
MATERIALS AND METHODS: Total 46 patients were registered and randomly allocated by computer generated chart by into three groups. In group A ( = 15), -based was applied in fistula-in-ano without any oral medication; in group B ( = 16), -based was applied with orally; and in group C ( = 15), -based was applied with orally. Patients were assessed for pain, discharge, itching and swelling in the affected region and unit cutting time (UCT) of fistulous tract. was changed by railroad technique on weekly based follow-up till complete healing of the tract occurred.
RESULTS: In group A, relief in pain, discharge, and swelling was found and was statistically highly significant while insignificant result was found in itching after cut through of the fistulous tract and the same results were found in group B ( = 14) and group C ( = 15). The mean UCT was higher in group A (8.94 days/cm) than in group C (8.43 days/cm) and in group B (8.59 days/cm).
CONCLUSION: based is more effective in cutting of fistula track while based is more effective in pain relief in the treatment of , along with oral as compared to based with and without orally.
Ayu. 2020;41(4):211-217.
PMID: 35813359 [PubMed - as supplied by publisher]
|
| 9. |
Herbal medicinal products for inflammatory bowel disease: A focus on those assessed in double-blind randomised controlled trials.
Holleran G, Scaldaferri F, Gasbarrini A, Currò D
Inflammatory bowel disease patients frequently use herbal products as complementary or alternative medicines to current pharmacotherapies and obtain information on them mainly from the internet, social media, or unlicensed practitioners. Clinicians should therefore take a more active role and become knowledgeable of the mechanisms of action and potential drug interactions of herbal medicines for which evidence of efficacy is available. The therapeutic efficacy and safety of several herbal medicines have been studied in double-blind randomised controlled trials (RCTs). Evidence of efficacy is available for Andrographis paniculata extract; curcumin; a combination of myrrh, extract of chamomile flower, and coffee charcoal; and the Chinese herbal medicines Fufangkushen colon-coated capsule and Xilei san in patients with ulcerative colitis; and Artemisia absinthium extract and Boswellia serrata resin extract in patients with Crohn's disease. However, most of this evidence comes from single small RCTs with short follow-up, and the long-term effects and safety of their use have not yet been established. Thus, our findings indicate that further appropriately sized RCTs are necessary prior to the recommended use of these herbal medicines in therapy. In the meantime, increasing awareness of their use, and potential drug interactions among physicians may help to reduce unwanted effects and adverse disease outcomes.
Phytother Res. 2020 Jan;34(1):77-93.
PMID: 31701598 [PubMed - indexed for MEDLINE]
|
| 10. |
Oral administration of a lecithin-based delivery form of boswellic acids (Casperome®) for the prevention of symptoms of irritable bowel syndrome: a randomized clinical study.
Riva A, Giacomelli L, Togni S, Franceschi F, Eggenhoffner R, Zuccarini MC, Belcaro G
BACKGROUND: The purpose of this study was to evaluate the long-term efficacy and the safety of a lecithin-based delivery form of boswellic acids from Boswellia serrata (Casperome®) for the prevention of symptoms in otherwise healthy subjects with mild irritable bowel syndrome (IBS).
METHODS: The study included 69 otherwise healthy participants with a mild form of IBS who completed a 6-month follow-up period. In total, 34 subjects were assigned to the standard management (SM) group: diet and, if needed, hyoscine butylbromide (Buscopan®) or papaverine hydrochloride + 10 mg of Atropa belladonna extract; 35 subjects were assigned to supplementation with the Boswellia serrata lecithin-based delivery form (one tablet/day; Casperome®). IBS signs and symptoms were evaluated at inclusion (T0), after 3 (T1) and 6 months (T2). The numbers of patients who needed rescue medication were recorded. Adverse events were also evaluated.
RESULTS: At baseline, the groups were comparable in terms of demographic and clinical characteristics. At follow-up, compared with the SM group, the Boswellia group showed lower mean score values for almost all self-assed IBS symptoms. A significantly lower need for rescue medications and consultations or medical evaluation/admissions was found in the Boswellia group compared with the SM group. The incidence of minimal adverse events - mainly stipsis - was significantly higher in the SM group. Oxidative stress at T2 was significantly decreased in Boswellia-supplemented subjects.
CONCLUSIONS: Boswellia serrata lecithin-based delivery form (Casperome®) appears to be effective and safe in improving signs and symptoms in IBS subjects who are otherwise healthy, particularly in comparison with symptomatic drug treatment that may cause side effects and stiptis.
Minerva Gastroenterol Dietol. 2019 Mar;65(1):30-35.
PMID: 30676012 [PubMed - indexed for MEDLINE]
|
| 11. |
Drug-Herb Interactions in the Elderly Patient with IBD: a Growing Concern.
Rahman H, Kim M, Leung G, Green JA, Katz S
Inflammatory bowel disease (IBD), which includes conditions such as Crohn's disease and ulcerative colitis, is becoming more prevalent with the elderly being the fastest growing group. Parallel to this, there is an increasing interest in the use of complementary and alternative medicine (CAM). Nearly half of patients with IBD have used CAM at one time. The elderly patients, however, are burdened by comorbid conditions, polypharmacy, and altered functional status. With increasing use of complementary and alternative medicine in our elderly patients with IBD, it is vital for the provider to provide counsel on drug-herb potential interactions. CAM includes herbal products, diet, dietary supplements, acupuncture, and prayer. In this paper, we will review common CAM, specifically herbs, that are used in patients with IBD including the herb background, suggested use, evidence in IBD, and most importantly, potential interactions with IBD medications used in elderly patients. Most important evidence-based adverse events and drug-herb interactions are summarized. The herbs discussed include Triticum aestivum (wheat grass), Andrographis paniculata (chiretta), Boswellia serrata, tormentil, bilberry, curcumin (turmeric), Plantago ovata (blond psyllium), Oenothera biennis (evening primrose oil), germinated barley foodstuff, an herbal preparation of myrrh, chamomile and coffee extract, chios mastic gum, wormwood (absinthe, thujone), Cannabis sativa (marijuana, THC), tripterygium wilfordii (thunder god vine), Ulmus rubra (slippery elm bark), trigonella foenugraecum (fenugreek), Dioscorea mexicana (wild yam), Harpagophytum procumbens (devil's claw), ginger, cinnamon, licorice, and peppermint.
Curr Treat Options Gastroenterol. 2017 Dec;15(4):618-636.
PMID: 28918484 [PubMed - as supplied by publisher]
|
| 12. |
Supplementation with a lecithin-based delivery form of Boswellia serrata extract (Casperome®) controls symptoms of mild irritable bowel syndrome.
Belcaro G, Gizzi G, Pellegrini L, Corsi M, Dugall M, Cacchio M, Feragalli B, Togni S, Riva A, Eggenhoffner R, Giacomelli L
OBJECTIVE: Irritable Bowel Syndrome (IBS) is a chronic, gastrointestinal disorder in which abdominal pain or discomfort is associated with defecation or changes in bowel habits. Its multifactorial pathophysiology leads to a variety of available treatments, mainly aimed at controlling symptoms. The management of IBS patients could be optimized by individualized strategies, including non-pharmaceutical approaches. In this study, we evaluated the efficacy and safety of a novel delivery form of Boswellia serrata extracts (BSE) (Casperome®) in patients with IBS.
PATIENTS AND METHODS: 71 otherwise healthy subjects with idiopathic IBS were recruited. Participants were assigned to the following management strategies: hyoscine butylbromide; papaverine hydrochloride + A. belladonna extract; supplementation with Casperome®. Predominant IBS symptoms were evaluated at inclusion and at the end of the observational period (4 weeks). The numbers of subjects who needed rescue medication or medical attention/hospital admission were recorded. Adverse events were also evaluated.
RESULTS: In all groups, the IBS symptoms investigated, namely abdominal pain, altered bowel movements, meteorism and cramps improved during the observational period. Of note, the number of subjects who needed medical attention significantly decreased only in Casperome®-supplemented group. In addition, Casperome® supplementation was related to a lower incidence of side effects (mainly stypsis).
CONCLUSIONS: This preliminary study suggests that Casperome® supplementation could represent a promising alternative approach to manage symptoms associated with IBS in otherwise healthy subjects.
Eur Rev Med Pharmacol Sci. 2017 May;21(9):2249-2254.
PMID: 28537656 [PubMed - indexed for MEDLINE]
|
| 13. |
Antioxidant Supplements and Gastrointestinal Diseases: A Critical Appraisal.
Khan I, Samson SE, Grover AK
The gastrointestinal tract digests and absorbs dietary nutrients, protects the body against physical and chemical damage from contents in its lumen, provides immunity against external antigens, and keeps an optimum environment for the gut microbiota. These functions cannot be performed normally in several diseases of which the following are discussed here: irritable bowel syndrome and inflammatory bowel disease, which includes Crohn's disease and ulcerative colitis. Because these diseases are associated with oxidative stress, a host of antioxidant supplements are used for maintenance and recovery of the gut functions. However, the benefits of these supplements have not been established. The available 80 human trials were rated for levels of confidence and for benefits of the antioxidant supplements. For Crohn's disease, the supplements for which clear benefits occurred in at least 2 studies were allopurinol, Boswellia serrata (frankincense or shallaki), Artemesia species (wormwood), Tripterygium wilfordii (léi gōng téng), and omega-3 fatty acids. Similar beneficial supplements for ulcerative colitis were allopurinol, Matricaria chamomilla (chamomile), Curcuma longa (curcumin in turmeric), and omega-3 fatty acids. There was also a clear benefit for ulcerative colitis in 2 studies where a multiherbal Chinese medicine preparation and an Ayurvedic medicine preparation were used. For irritable bowel syndrome, there was only a marginal benefit of some of the antioxidant supplements. Thus, some antioxidant supplements may be beneficial at certain stages of specific diseases. This is consistent with the current concept that antioxidants act by inhibiting oxidative stress pathways in a tissue- and environment-specific manner and not by simply acting as scavengers.
Med Princ Pract. 2017;26(3):201-217.
PMID: 28278495 [PubMed - indexed for MEDLINE]
|
| 14. |
The role of dietary supplements in inflammatory bowel disease: a systematic review.
Rossi RE, Whyand T, Murray CD, Hamilton MI, Conte D, Caplin ME
Inflammatory bowel diseases (IBD) are chronic immune disorders of unclear aetiology. Dietary deficiencies may be a potential pathogenic factor in their development. Patients often take food supplements without knowledge of any evidence base. We have therefore assessed the evidence for food supplementation in the management of IBD. A PubMed search was performed for the terms Inflammatory bowel disease; nutritional deficiencies; dietary supplements; curcumin; green tea; vitamin D/other vitamins; folic acid; iron; zinc; probiotics; andrographis paniculata; and boswellia serrate. PubMed was used to search for all relevant articles published between January 1975 and September 2015. Curcumin supplementation has been reported to be effective in reducing the symptoms and the inflammatory indices in IBD patients. Similar results have been observed for green tea; however, pertinent studies are limited. Vitamin D supplementation may help to increase bone mineral density in IBD patients and to reduce disease activity. IBD patients with ileal resections higher than 20 cm may develop vitamin B12 deficiency that requires parenteral supplementation. There is no current evidence to support fat-soluble vitamin supplementation in IBD patients. Zinc and iron should be supplemented in selected cases. Probiotics (VSL#3) may reduce disease activity in IBD patients with pouchitis. Complementary and alternative medicines are used by IBD patients and some studies have shown promising results. In summary, attention to dietary factors such as curcumin, green tea and vitamins, including vitamins D and B12, appears to be beneficial and, if necessary, supplementation may be appropriate.
Eur J Gastroenterol Hepatol. 2016 Dec;28(12):1357-1364.
PMID: 27769076 [PubMed - indexed for MEDLINE]
|
| 15. |
Managing ulcerative colitis in remission phase: usefulness of Casperome®, an innovative lecithin-based delivery system of Boswellia serrata extract.
Pellegrini L, Milano E, Franceschi F, Belcaro G, Gizzi G, Feragalli B, Dugall M, Luzzi R, Togni S, Eggenhoffner R, Giacomelli L
OBJECTIVE: Boswellia serrata extracts (BSE) have been traditionally used for the treatment of several inflammatory diseases. The aim of this study was to evaluate the efficacy of a novel delivery form of BSE (Casperome®) in Ulcerative Colitis (UC) during minimally symptomatic remission phase.
PATIENTS AND METHODS: In this open-label, observational, registry study, informed participants with UC in remission phase (n = 43) freely decided to receive the oral daily Casperome® supplementation (n = 22) or no supplementation (n = 21) for 4 weeks. Several parameters associated with minimally symptomatic UC in remission were evaluated at the inclusion and the end of the study.
RESULTS: A significant beneficial effect of Casperome® was observed for all the parameters evaluated, namely: diffuse intestinal pain, evident and occult blood in stools, bowel movements and cramps, watery stools, malaise, anemia, rectal involvement, number of white blood cells as well as need for concomitant drugs and medical attention. Faecal concentration of calprotectin, a marker of bowel inflammation, resulted ameliorated in Casperome® supplemented patients.
CONCLUSIONS: Our study showed that Casperome® supplementation attenuates symptoms associated with mild UC in remission, reducing the use of drugs and medical consultations. Therefore, our study suggests that Casperome® supplementation could represent a promising alternative approach to manage minimally symptomatic UC and maintain the remission phase.
Eur Rev Med Pharmacol Sci. 2016 Jun;20(12):2695-700.
PMID: 27383325 [PubMed - indexed for MEDLINE]
|
| 16. |
Dietary Supplement Therapies for Inflammatory Bowel Disease: Crohn's Disease and Ulcerative Colitis.
Parian A, Limketkai BN
Inflammatory bowel disease (IBD) including ulcerative colitis and Crohn's disease are chronic relapsing and remitting chronic diseases for which there is no cure. The treatment of IBD frequently requires immunosuppressive and biologic therapies which carry an increased risk of infections and possible malignancy. There is a continued search for safer and more natural therapies in the treatment of IBD. This review aims to summarize the most current literature on the use of dietary supplements for the treatment of IBD. Specifically, the efficacy and adverse effects of vitamin D, fish oil, probiotics, prebiotics, curcumin, Boswellia serrata, aloe vera and cannabis sativa are reviewed.
Curr Pharm Des. 2016;22(2):180-8.
PMID: 26561079 [PubMed - indexed for MEDLINE]
|
| 17. |
Medical Plant Extracts for Treating Knee Osteoarthritis: a Snapshot of Recent Clinical Trials and Their Biological Background.
Walzer SM, Weinmann D, Toegel S
In light of the growing global health problem associated with osteoarthritis, herbal remedies have become an important research focus in the scientific and medical community, and numerous studies have been published to identify their biological effects and mechanisms in vitro and in vivo. This review is a snapshot of the most recent clinical trials on the efficacy of medical plant extracts in knee osteoarthritis patients, and provides relevant background information on the biological mechanisms that may underlie the clinical observations. Therefore, we performed a PubMed literature survey and discussed a selection of clinical trials in the field, with special attention being drawn to the design and outcome measures of the studies. We further spotlighted on issues relating to the efficacy and safety of the plant extracts and discussed major challenges for upcoming studies in the field, which include the need for rigorously designed in vivo and in vitro studies, as well as the elucidation of potential additive effects and structure-modifying activities beyond symptom relief.
Curr Rheumatol Rep. 2015 Aug;17(8):54.
PMID: 26163305 [PubMed - indexed for MEDLINE]
|
| 18. |
Nutraceutical Supplements for Inflammatory Bowel Disease.
Parian AM, Limketkai BN, Shah ND, Mullin GE
Nutr Clin Pract. 2015 Aug;30(4):551-8.
PMID: 26024677 [PubMed - indexed for MEDLINE]
|
| 19. |
Herbal and plant therapy in patients with inflammatory bowel disease.
Triantafyllidi A, Xanthos T, Papalois A, Triantafillidis JK
The use of herbal therapy in inflammatory bowel disease (IBD) is increasing worldwide. The aim of this study was to review the literature on the efficacy of herbal therapy in IBD patients. Studies on herbal therapy for IBD published in Medline and Embase were reviewed, and response to treatment and remission rates were recorded. Although the number of the relevant clinical studies is relatively small, it can be assumed that the efficacy of herbal therapies in IBD is promising. The most important clinical trials conducted so far refer to the use of mastic gum, tormentil extracts, wormwood herb, , , germinated barley foodstuff, and . In ulcerative colitis, gel, riticum aestivum, andrographis paniculata extract and topical Xilei-san were superior to placebo in inducing remission or clinical response, and curcumin was superior to placebo in maintaining remission; gum resin and seeds were as effective as mesalazine, whereas oenothera biennis had similar relapse rates as ω-3 fatty acids in the treatment of ulcerative colitis. In Crohn's disease, mastic gum, , and were superior to placebo in inducing remission and preventing clinical postoperative recurrence, respectively. Herbal therapies exert their therapeutic benefit by different mechanisms including immune regulation, antioxidant activity, inhibition of leukotriene B4 and nuclear factor-kappa B, and antiplatelet activity. Large, double-blind clinical studies assessing the most commonly used natural substances should urgently be conducted.
Ann Gastroenterol. 2015;28(2):210-220.
PMID: 25830661 [PubMed - as supplied by publisher]
|
| 20. |
Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases.
Langhorst J, Wulfert H, Lauche R, Klose P, Cramer H, Dobos GJ, Korzenik J
OBJECTIVE: We performed a systematic review for Complementary and Alternative Medicine [CAM] as defined by the National Institute of Health in Inflammatory Bowel Disease [IBD], ie Crohn's disease [CD] and ulcerative colitis [UC], with the exception of dietary and nutritional supplements, and manipulative therapies.
METHODS: A computerized search of databases [Cochrane Library, Pubmed/Medline, PsychINFO, and Scopus] through March 2014 was performed. We screened the reference sections of original studies and systematic reviews in English language for CAM in IBD, CD and UC. Randomized controlled trials [RCT] and controlled trials [CT] were referred and assessed using the Cochrane risk of bias tool.
RESULTS: A total of: 26 RCT and 3 CT for herbal medicine, eg aloe-vera gel, andrographis paniculata, artemisia absinthium, barley foodstuff, boswellia serrata, cannabis, curcumin, evening primrose oil, Myrrhinil intest®, plantago ovata, silymarin, sophora, tormentil, wheatgrass-juice and wormwood; 1 RCT for trichuris suis ovata; 7 RCT for mind/body interventions such as lifestyle modification, hypnotherapy, relaxation training and mindfulness; and 2 RCT in acupuncture; were found. Risk of bias was quite heterogeneous. Best evidence was found for herbal therapy, ie plantago ovata and curcumin in UC maintenance therapy, wormwood in CD, mind/body therapy and self-intervention in UC, and acupuncture in UC and CD.
CONCLUSIONS: Complementary and alternative therapies might be effective for the treatment of inflammatory bowel diseases; however, given the low number of trials and the heterogeneous methodological quality of trials, further in-depth research is necessary.
J Crohns Colitis. 2015 Jan;9(1):86-106.
PMID: 25518050 [PubMed - indexed for MEDLINE]
|
| 21. |
Complementary and alternative medicine in inflammatory bowel diseases: what is the future in the field of herbal medicine?
Gilardi D, Fiorino G, Genua M, Allocca M, Danese S
The use of complementary and alternative medicine is wide-spread not only in Eastern countries, but also in the Western world. Despite the increasing evidence on the harmful effects induced by several naturopathic/homeopathic products, patients seem to appreciate these remedies, in particular because they consider them to be absolutely safe. This same phenomenon is common among inflammatory bowel disease (IBD) patients. As a result there is a significant request for scientific data to evaluate both the efficacy and safety of these remedies, and to support the use of such medications as adjuvant treatments to biological and synthetic drugs. We aimed to review the current evidence on efficacy and safety of some natural products that are believed to be effective in inflammatory bowel disease. Further perspectives for the clinical use of herbal products and strategies for improving knowledge about herbal products in IBD are also discussed.
Expert Rev Gastroenterol Hepatol. 2014 Sep;8(7):835-46.
PMID: 24813226 [PubMed - indexed for MEDLINE]
|
| 22. |
Induction of clinical response and remission of inflammatory bowel disease by use of herbal medicines: a meta-analysis.
Rahimi R, Nikfar S, Abdollahi M
AIM: To evaluate the efficacy and tolerability of herbal medicines in inflammatory bowel disease (IBD) by conducting a meta-analysis.
METHODS: Electronic databases were searched for studies investigating efficacy and/or tolerability of herbal medicines in the management of different types of IBD. The search terms were: "herb" or "plant" or "herbal" and "inflammatory bowel disease". Data were collected from 1966 to 2013 (up to Feb). The "clinical response", "clinical remission", "endoscopic response", "endoscopic remission", "histological response", "histological remission", "relapse", "any adverse events", and "serious adverse events" were the key outcomes of interest. We used the Mantel-Haenszel, Rothman-Boice method for fixed effects and DerSimonian-Laird method for random-effects. For subgroup analyses, we separated the studies by type of IBD and type of herbal medicine to determine confounding factors and reliability.
RESULTS: Seven placebo controlled clinical trials met our criteria and were included (474 patients). Comparison of herbal medicine with placebo yielded a significant RR of 2.07 (95%CI: 1.41-3.03, P = 0.0002) for clinical remission; a significant RR of 2.59 (95%CI: 1.24-5.42, P = 0.01) for clinical response; a non-significant RR of 1.33 (95%CI: 0.93-1.9, P = 0.12) for endoscopic remission; a non-significant RR of 1.69 (95%CI: 0.69-5.04) for endoscopic response; a non-significant RR of 0.64 (95%CI: 0.25-1.81) for histological remission; a non-significant RR of 0.86 (95%CI: 0.55-1.55) for histological response; a non-significant RR of 0.95 (95%CI: 0.52-1.73) for relapse; a non-significant RR of 0.89 (95%CI: 0.75-1.06, P = 0.2) for any adverse events; and a non-significant RR of 0.97 (95%CI: 0.37-2.56, P = 0.96) for serious adverse events.
CONCLUSION: The results showed that herbal medicines may safely induce clinical response and remission in patients with IBD without significant effects on endoscopic and histological outcomes, but the number of studies is limited to make a strong conclusion.
World J Gastroenterol. 2013 Sep;19(34):5738-49.
PMID: 24039370 [PubMed - indexed for MEDLINE]
|
| 23. |
Systematic review: the efficacy of herbal therapy in inflammatory bowel disease.
Ng SC, Lam YT, Tsoi KK, Chan FK, Sung JJ, Wu JC
BACKGROUND: Complementary and alternative medicine (CAM), particularly herbal therapy, is widely used by patients with inflammatory bowel disease (IBD) but controlled data are limited.
AIM: To systematically review the literature on the efficacy of herbal therapy in the treatment of ulcerative colitis (UC) and Crohn's disease (CD).
METHODS: Publications in English and non-English literatures (MEDLINE, EMBASE, EBM Reviews, AMED, Global Health) were searched from 1947 to 2013 for controlled clinical studies of herbal therapy in IBD. Outcome measures included response and remission rates.
RESULTS: Twenty-one randomised controlled trials (14 UC; 7 CD) including a total of 1484 subjects (mean age 41, 50% female) were analysed. In UC, aloe vera gel, Triticum aestivum (wheat grass juice), Andrographis paniculata extract (HMPL-004) and topical Xilei-san were superior to placebo in inducing remission or response, and curcumin was superior to placebo in maintaining remission; Boswellia serrata gum resin and Plantago ovata seeds were as effective as mesalazine, whereas Oenothera biennis (evening primrose oil) had similar relapse rates as omega-3 fatty acids in the treatment of UC. In CD, Artemisia absinthium (wormwood) and Tripterygium wilfordii were superior to placebo in inducing remission, and preventing clinical recurrence of post-operative CD respectively.
CONCLUSIONS: Randomised controlled trials of herbal therapy for the treatment of IBD show encouraging results but studies remain limited and heterogenous. Larger controlled studies with stricter endpoints and better-defined patient groups are required to obtain more conclusive results on the use of CAM therapies in IBD.
Aliment Pharmacol Ther. 2013 Oct;38(8):854-63.
PMID: 23981095 [PubMed - indexed for MEDLINE]
|
| 24. |
Randomized, placebo-controlled, double-blind trial of Boswellia serrata in maintaining remission of Crohn's disease: good safety profile but lack of efficacy.
Holtmeier W, Zeuzem S, Preiss J, Kruis W, Böhm S, Maaser C, Raedler A, Schmidt C, Schnitker J, Schwarz J, Zeitz M, Caspary W
BACKGROUND: Complementary therapies are frequently used by patients with inflammatory bowel disease (IBD). The aim of this study was to evaluate the efficacy and safety of long-term therapy with a new Boswellia serrata extract (Boswelan, PS0201Bo) in maintaining remission in patients with Crohn's disease (CD).
METHODS: In 22 German centers a double-blind, placebo-controlled, randomized, parallel study was performed. In all, 108 outpatients with CD in clinical remission were included. Patients were randomized to Boswelan (3×2 capsules/day; 400 mg each) or placebo for 52 weeks. The primary endpoint was the proportion of patients in whom remission was maintained throughout the 52 weeks. Secondary endpoints were time to relapse, changes of Crohn's Disease Activity Index (CDAI), and IBD Questionnaire (IBDQ) scores.
RESULTS: The trial was prematurely terminated due to insufficient discrimination of drug and placebo with regard to the primary efficacy endpoint. A total of 82 patients were randomized to Boswelan (n=42) or placebo (n=40). Sixty-six patients could be analyzed for efficacy. 59.9% of the actively treated patients and 55.3% of the placebo group stayed in remission (P=0.85). The mean time to diagnosis of relapse was 171 days for the active group and 185 days for the placebo group (P=0.69). With respect to CDAI, IBDQ, and laboratory measurements of inflammation, no advantages in favor of active treatment were detected. Regarding safety concerns, no disadvantages of taking the drug compared to placebo were observed.
CONCLUSIONS: The trial confirmed good tolerability of a new Boswellia serrata extract, Boswelan, in long-term treatment of CD. However, superiority versus placebo in maintenance therapy of remission could not be demonstrated.
Inflamm Bowel Dis. 2011 Feb;17(2):573-82.
PMID: 20848527 [PubMed - indexed for MEDLINE]
|
| 25. |
Effect of exclusion diet with nutraceutical therapy in juvenile Crohn's disease.
Slonim AE, Grovit M, Bulone L
BACKGROUND: Most moderate-severe juvenile Crohn's disease (CD) patients are in a constant catabolic state resulting in poor weight gain and growth failure. Anti-inflammatory, immunomodulatory, and monoclonal antibody drugs, as well as growth hormone (GH), frequently fail to achieve sustained remission or reverse growth failure.
OBJECTIVE: To test whether an exclusion diet with nutraceutical therapy (DNT) could induce sustained clinical remission and weight gain, and if so does this enhance the ability for GH to reverse growth failure.
METHODS: An uncontrolled prospective case study was undertaken in six moderate- severe CD patients, two of whom had completed growth. All were treated with DNT. Adequate caloric and protein ( >or= 3g/kg/d) intake for catch up weight was prescribed. Dairy products, certain grains and carrageenan containing foods were eliminated. Nutraceuticals, consisting of fish peptides, bovine colostrum, boswellia serrata, curcumin and a multivitamin were administered daily. Lactobacillus GG, a probiotic, was administered twice weekly. Recombinant human GH (rhGH) was administered daily.
RESULTS: Within 2 months of starting DNT all six patients went into remission, with discontinuation of all pharmacological drugs. Three patients have remained in sustained remission for 4 to 8 years. One patient with very severe CD had recurrence of CD symptoms after being in complete remission for 18 months, one patient was in remission for 3 years but symptoms recurred when she became less compliant to DNT and one recently treated patient remains in remission after 6 months. With the addition of rhGH, the 4 growing patients had good-excellent growth response
CONCLUSION: DNT engendered prolonged remission and restoration of normal weight in moderate-severe juvenile CD patients, providing conditions that enabled rhGH to stimulate growth. These findings justify larger controlled trials to evaluate the long-term benefit of compliance to DNT in both juvenile and adult CD patients.
J Am Coll Nutr. 2009 Jun;28(3):277-85.
PMID: 20150601 [PubMed - indexed for MEDLINE]
|
| 26. |
Boswellia serrata extract for the treatment of collagenous colitis. A double-blind, randomized, placebo-controlled, multicenter trial.
Madisch A, Miehlke S, Eichele O, Mrwa J, Bethke B, Kuhlisch E, Bästlein E, Wilhelms G, Morgner A, Wigginghaus B, Stolte M
BACKGROUND AND AIMS: The objective of this study was to investigate the effect of Boswellia serrata extract (BSE) on symptoms, quality of life, and histology in patients with collagenous colitis.
MATERIALS AND METHODS: Patients with chronic diarrhea and histologically proven collagenous colitis were randomized to receive either oral BSE 400 mg three times daily for 6 weeks or placebo. Complete colonoscopy and histology were performed before and after treatment. Clinical symptoms and quality of life were assessed by standardized questionnaires and SF-36. The primary endpoint was the percentage of patients with clinical remission after 6 weeks (stool frequency
RESULTS: Thirty-one patients were randomized; 26 patients were available for per-protocol-analysis. After 6 weeks, the proportion of patients in clinical remission was higher in the BSE group than in the placebo group (per protocol 63.6%; 95%CI, 30.8-89.1 vs 26.7%, 95%CI, 7.7-55.1; p=0.04; intention-to-treat 43.8% vs 26.7%, p=0.25). Compared to placebo, BSE treatment had no effect on histology and quality of life. Five patients discontinued BSE treatment prematurely. Discontinuation was due to adverse events (n=1), unwillingness to continue (n=3), or loss to follow-up for unknown reasons (n=1). Seven patients received open-label BSE therapy, five of whom achieved complete remission.
CONCLUSIONS: Our study suggests that BSE might be clinically effective in patients with collagenous colitis. Larger trials are clearly necessary to establish the clinical efficacy of BSE.
Int J Colorectal Dis. 2007 Dec;22(12):1445-51.
PMID: 17764013 [PubMed - indexed for MEDLINE]
|
| 27. |
Use of complementary and alternative medicine in Germany - a survey of patients with inflammatory bowel disease.
Joos S, Rosemann T, Szecsenyi J, Hahn EG, Willich SN, Brinkhaus B
BACKGROUND: Previous studies have suggested an increasing use of complementary and alternative medicine (CAM) in patients with inflammatory bowel disease (IBD). The aim of our study was to evaluate the use of CAM in German patients with IBD.
METHODS: A questionnaire was offered to IBD patients participating in patient workshops which were organized by a self-help association, the German Crohn's and Colitis Association. The self-administered questionnaire included demographic and disease-related data as well as items analysing the extent of CAM use and satisfaction with CAM treatment. Seven commonly used CAM methods were predetermined on the questionnaire.
RESULTS: 413 questionnaires were completed and included in the analysis (n = 153 male, n = 260 female; n = 246 Crohn's disease, n = 164 ulcerative colitis). 52 % of the patients reported CAM use in the present or past. In detail, homeopathy (55%), probiotics (43%), classical naturopathy (38%), Boswellia serrata extracts (36%) and acupuncture/Traditional Chinese Medicine (TCM) (33%) were the most frequently used CAM methods. Patients using probiotics, acupuncture and Boswellia serrata extracts (incense) reported more positive therapeutic effects than others. Within the statistical analysis no significant predictors for CAM use were found. 77% of the patients felt insufficiently informed about CAM.
CONCLUSION: The use of CAM in IBD patients is very common in Germany, although a large proportion of patients felt that information about CAM is not sufficient. However, to provide an evidence-based approach more research in this field is desperately needed. Therefore, physicians should increasingly inform IBD patients about benefits and limitations of CAM treatment.
BMC Complement Altern Med. 2006 May;6():19.
PMID: 16716218 [PubMed - indexed for MEDLINE]
|
| 28. |
Effects of gum resin of Boswellia serrata in patients with chronic colitis.
Gupta I, Parihar A, Malhotra P, Gupta S, Lüdtke R, Safayhi H, Ammon HP
Patients studied here suffered from chronic colitis characterized by vague lower abdominal pain, bleeding per rectum with diarrhoea and palpable tender descending and sigmoid colon. The inflammatory process in colitis is associated with increased formation of leukotrienes causing chemotaxis, chemokinesis, synthesis of superoxide radicals and release of lysosomal enzymes by phagocytes. The key enzyme for leukotriene biosynthesis is 5-lipoxygenase. Boswellic acids were found to be non-redox, non-competitive specific inhibitors of the enzyme 5-lipoxygenase. We studied the gum resin of Boswellia serrata for the treatment of this disease. Thirty patients, 17 males and 13 females in the age range of 18 to 48 years with chronic colitis were included in this study. Twenty patients were given a preparation of the gum resin of Boswellia serrata (900 mg daily divided in three doses for 6 weeks) and ten patients were given sulfasalazine (3 gm daily divided in three doses for 6 weeks) and served as controls. Out of 20 patients treated with Boswellia gum resin 18 patients showed an improvement in one or more of the parameters: including stool properties, histopathology as well as scanning electron microscopy, besides haemoglobin, serum iron, calcium, phosphorus, proteins, total leukocytes and eosinophils. In the control group 6 out of 10 patients showed similar results with the same parameters. Out of 20 patients treated with Boswellia gum resin 14 went into remission while in case of sulfasalazine remission rate was 4 out of 10. In conclusion, this study shows that a gum resin preparation from Boswellia serrata could be effective in the treatment of chronic colitis with minimal side effects.
Planta Med. 2001 Jul;67(5):391-5.
PMID: 11488449 [PubMed - indexed for MEDLINE]
|
| 29. |
[Therapy of active Crohn disease with Boswellia serrata extract H 15].
Gerhardt H, Seifert F, Buvari P, Vogelsang H, Repges R
BACKGROUND: The purpose of this clinical trial was to compare efficacy and safety of the Boswellia serrata extract H15 with mesalazine for the treatment of active Crohn's disease.
PATIENTS AND METHODS: Randomised, double-blind, verum-controlled, parallel group comparison for which 102 Patients were randomised. The per protocol population included 44 patients treated with H15 and 39 patients treated with mesalazine. As primary outcome measure the change of the Crohn Disease Activity Index (CDAI) between the status of enrolment and end of therapy was chosen. H 15 was tested on non-inferiority compared to standard treatment with mesalazine.
RESULTS: The CDAI between the status of enrolment and end of therapy after treatment with H15 was reduced by 90 and after therapy with mesalazine by 53 scores in the mean. In this non-inferiority-trial the test hypothesis was confirmed by the statistical analysis. The difference between both treatments could not be proven to be statistically significant in favor to H15 for the primary outcome measure. The secondary efficacy endpoints confirm the assessment of the comparison of H15 and mesalazine. The proven tolerability of H15 completes the results of the shown clinical efficacy.
CONCLUSIONS: The study confirms that therapy with H15 is not inferior to mesalazine. This can be interpreted as evidence for the efficacy of H15 according to the state of art in the treatment of active Crohn's disease with Boswellia serrata extract, since the efficacy of mesalazine for this indication has been approved by the health authorities. Considering both safety and efficacy of Boswellia serrata extract H15 it appears to be superior over mesalazine in terms of a benefit-risk-evaluation.
Z Gastroenterol. 2001 Jan;39(1):11-7.
PMID: 11215357 [PubMed - indexed for MEDLINE]
|
| 30. |
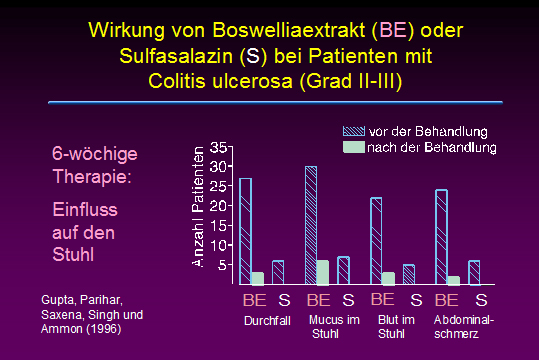
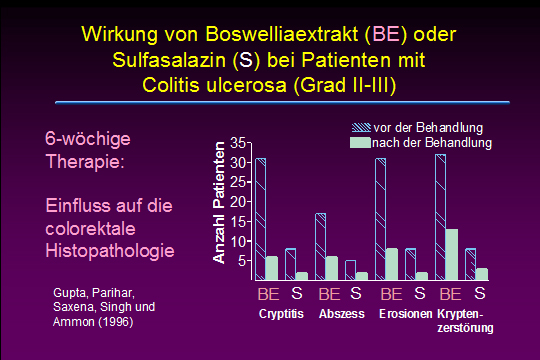
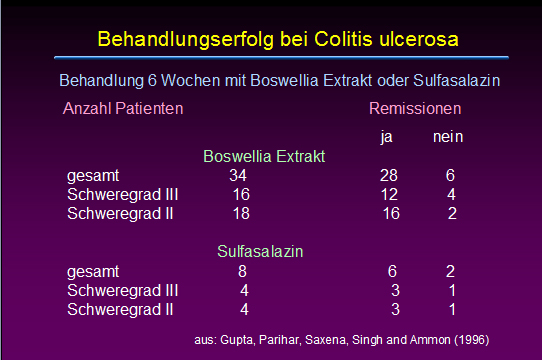
Effects of Boswellia serrata gum resin in patients with ulcerative colitis.
Gupta I, Parihar A, Malhotra P, Singh GB, Lüdtke R, Safayhi H, Ammon HP
Ulcerative colitis is a chronic inflammatory disease of the colon where leukotrienes are suggested to play an important role for keeping inflammation active. Boswellic acids, the biologically active ingredients of the gum resin of Boswellia serrata (Sallai guggal), have been shown to be specific, nonredox and noncompetitive inhibitors of 5-lipoxygenase, the key enzyme of leukotriene biosynthesis. In patients suffering from ulcerative colitis grade II and III the effect of Boswellia serrata gum resin preparation (350 mg thrice daily for 6 weeks) on stool properties, histolopathology and scan microscopy of rectal biopsies, blood parameters including Hb, serum iron, calcium, phosphorus, proteins, total leukocytes and eosinophils was studied. Patients receiving sulfasalazine (1 g thrice daily) served as controls. All parameters tested improved after treatment with Boswellia serrata gum resin, the results being similar compared to controls: 82% out of treated patients went into remission; in case of sulfasalazine remission rate was 75%.
Eur J Med Res. 1997 Jan;2(1):37-43.
PMID: 9049593 [PubMed - indexed for MEDLINE]
|